Dear All (wonkish alert … get your coffee!),
EFPIA have recently released a report estimating the value of a Transferable Exclusivity Extension (TEE, report, press release) that provides new generalized insights into ways to think about the value of new antibiotics and the appropriate size of the Pull rewards needed to bring that new antibiotic into existence. To best appreciate the report, a bit of background is needed. Here we go…
Estimating the needed size of Pull reward (if Pull is new to you, see this fabulous 5-minute video explainer) requires us to balance the cost of creating and deploying a new antibiotic vs. its value to society. We have a best guess estimate of $1.7b for the all-in development and maintenance cost of a new antibiotic (from discovery to Year 10 on the market) and we have Kevin Outterson’s excellent and detailed work estimating that a fully delinked global subscription needs to be ~$3.1 billion (range: $2.2-4.8b) to drive innovation (Health Affairs. 2021;40(11):1758-65; https://doi.org/10.1377/hlthaff.2021.00688; and for completeness, note that a partially delinked subscription needs to have a global value of $1.6b; range $0.9-2.6b).
So, what then is the value to society? Here we must consider the “fire extinguisher value” of the existence of the antibiotic as protective/enabling infrastructure. Early estimates (e.g., Sertkaya 2014) have been progressively improved / critiqued and we now talk in terms of the “fire extinguisher value” idea that was analyzed in Rothery et al. 2018 as the STEDI attributes of new antibiotics: Spectrum, Transmission, Enablement, Diversity, and Insurance.
Excitingly, elements of STEDI were used by the UK NHS pilot group to estimate QALYs (Quality-Adjusted Life Years) and (implicitly) monetary value as the basis for the maiden voyage of the first antibacterial pull model. This subscription model will ensure access to two new antibacterial agents at a cost/drug to the UK of £10m/year. Based mostly on estimates of Enablement and Insurance value, the annual QALY values to the NHS for the compounds are 530 QALY/year and 970 QALY/year. As NICE typically uses £20,000 and £30,000 per QALY as a threshold for the idea of good value (that is, things that cost more than this per QALY are too expensive), a simple scaling of these QALYs/year x £20k/QALY = £11-19M/year, a value that exceeds the per drug subscription cost.
So now we come to the new report. It should be considered from two perspectives (i) its discussion of the idea of a TEE and (ii) the way the report adds new STEDI-based analyses to its cost-benefit math. For our discussion, let’s consider these in reverse order from the discussion in the report.
Part 1: How hard should we Pull? What is the value of a new antibiotic?
The major leap forward in this report is that its value estimations represent a new and generalizable build on prior work. Taking the STEDI concept as a framework and adding estimates of the value of clinical benefit of treating patients as well as productivity gains from enabling patients to get back to work, the authors use QALY-based approaches to estimate the value to society for 6 different countries of a new antibiotic over 10 years:
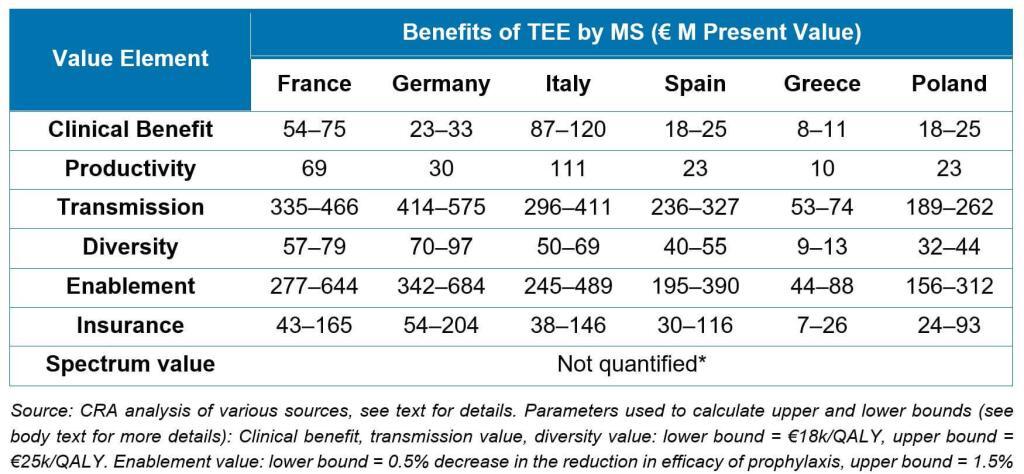
Notably, these analyses extend the value estimation work done for the NHS Pilot and are completely generalizable for any Pull mechanism.
As an added step, the authors show how the math can be made specific to different types of antibiotics. For example, the value of an antibiotic for fluoroquinolone-resistant Salmonella would predominantly arise from insurance value (i.e., on hand for an outbreak of food-borne infections), whereas the value of a new agent for carbapenem-resistant Acinetobacter would be in the critical care setting and predominantly driven by the clinical and productivity benefits, transmission value, and insurance value.
This work is an important step forward and will invite others to consider ways to extend the calculation for other countries and scenarios.
Part 2: How should we Pull? The idea of a TEE, especially for the EU
TEEs go by several names with TEV (Transferrable Exclusivity Voucher) and “wildcard patents” sometimes used to describe the concept. A TEE gives the developer of an eligible and novel antibiotic a voucher that can be used to extend the exclusivity (the period of protected sales) of another medicine for a defined (limited) period of time. The right to the TEE can be used by the developer or sold to another company. Each of those highlighted phrases has meaning and the net effect is that a TEE pays only for successful research that creates an interesting new antibiotic. The report from EFPIA argues that a TEE-based approach is a pragmatic approach to having a common EU-wide Pull mechanism:
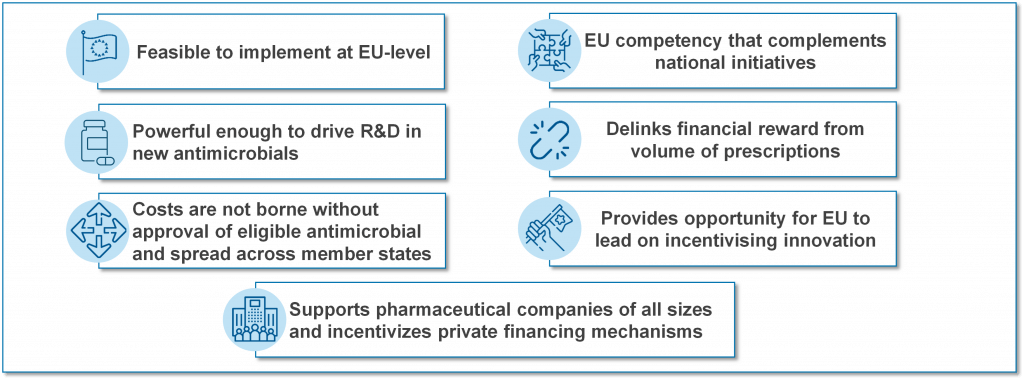
TEEs have a straightforward and appealing simplicity but can also be criticized because they transfer the cost of the antibiotic to the patients who need other drugs and the value of the TEE may be hard to control. Both of these concerns can be mitigated (these same patients would benefit from access to the new antibiotic and it would be possible to implement guardrails that constrain the amount earned from a TEE), however the lack of a simple way to estimate, manage, and restrict the value of the TEE has (in my view) been the main reason for favoring the clarity of the pre-defined value of a market entry reward approach (e.g., the UK NHS pilot).
To address this concern, the report includes calculations showing that the most likely actual by-country cost of the TEE will be less less than the value of having the antibiotic. The math in the report assumes that TEEs would have a length of 9-12 months, that at most 1-2 of them would be active at any given time, and that the main costs would be from administrative overhead plus lost generic savings due to extended exclusivity. In the figure below, the bars are the STEDI-based value components and the lines represent the estimated costs of 1 or 2 TEEs per year:
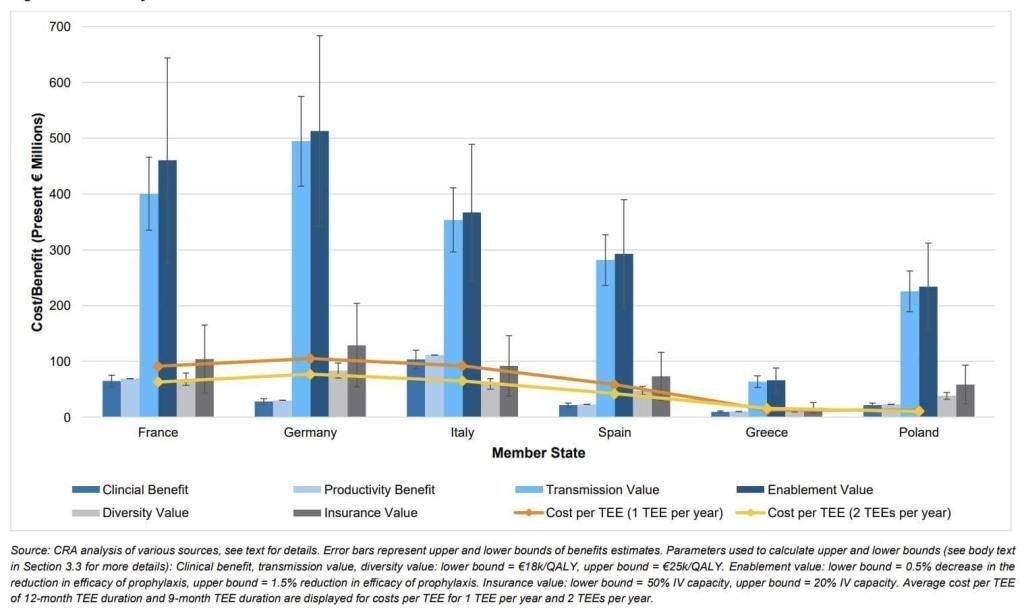
Overall, I think the report makes a good case that the value of the new antibiotic exceeds the cost of the TEE no matter how you choose to add up the value components. It is also notable that a TEE makes use of tools that are readily within the competency of the EU, thereby avoiding the difficulties of nation-by-nation passage of market entry reward-style Pull incentives across all 27 Member States.
For more on TEEs, see DRIVE-AB’s final report, Outterson & McDonnell (Health Affairs 2016), Seabury & Sood (Health Affairs 2017), Berdud et al. (OHE White Paper 2019), Rome & Kesselheim (CID 2020), Boyer et al. (2022 Duke Margolis White Paper). The risk that the financial value of the TEE will not be well balanced vs. the social value of the new antibiotic is a general theme in these papers, and the 2016 commentary by Outterson & McDonnell and the 2019 OHE paper by Berdud et al. both offer concrete ideas on ways that TEEs could be tailored to address concerns about financial mismatch.
—
Wow … a lot to consider! Many thanks to the authors of this report for making an effort to advance our critical thinking about the value of new antibiotics. No matter whether the Pull mechanism is a TEE or a Market Entry Reward, the value-based thinking in this report is an important next step in our thinking about the value of new antibiotics. And the arguments for a TEE are a welcome addition to the literature. Although you can criticize TEEs, feasibility is important: critiques of TEEs as a solution for Europe need to propose an alternative that could be implemented in non-geologic time!
In summary, “How (and How Hard) to Pull?” is not a simple question to answer! But make no mistake about it — as is clear from the plot of The Third Man, the right antibiotic can be worth more than gold!
How much is an antibiotic worth? Enough to provoke serious crime! Check it out!
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Other materials considered while writing today:
- Dubois P, de Mouzon O, Scott-Morton F, Seabright P. Market size and pharmaceutical innovation. RAND J Econ. 2015;46(4):84471. https://doi.org/10.1111/1756-2171.12113
- US National Academies of Science, Engineering, and Medicine: Combating Antimicrobial Resistance and Protecting the Miracle of Modern Medicine: This report by the Committee on the Long-Term Health and Economic Effects of Antimicrobial Resistance in the United States has (no surprise!) a strong focus on the cost of AMR. In particular, the paper’s Table 3-2 takes us back to the CDC’s 2019 report on AMR threat pathogens and finds that the total of the direct and attributable costs of the AMR threat pathogens to the US was over $10 billion in 2017!
- 2020 United States Government Accountability Office, Antibiotic Resistance: Additional Federal Actions Needed to Better Determine Magnitude and Reduce Impact.
- And see also the other references listed on https://amr.solutions/incentives/
Current funding opportunities (most current list is here)
- [NEW – SHORT TIMELINE}: RFP entitled “Building Capacity to Address Health Disparities in the United States through Antimicrobial Stewardship Telehealth & Tele-mentoring.” Sponsored by the Pfizer Global Medical Grants program, the title spells out the scope really well. Go here to apply; deadline is 6 Oct 2022.Queries can be sent to Jessica Romano, Grant Officer (jessica.romano@pfizer.com).
- The AMR Action Fund is now open to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- New funding rounds from CARB-X are expected soon now that funding for the next 10 years has been announced! For the most current update, watch this 30-minute video from the June 2022 kick-off webinar.
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- 4-7 Oct 2022 (Dublin, Ireland): The 2022 ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. This is an excellent meeting, especially for developers … and if you’ve missed it, the recordings from the 2021 meeting are online. Go here for details on the 2022 meeting.
- 13-14 Oct 2022 (virtual, 8.30a-5p ET). Workshop entitled “Accelerating the Development & Uptake of Rapid Diagnostics to Address Antibiotic Resistance.” Convened by the National Academies’ Forum on Drug Discovery, Development, and Translation, Forum on Medical and Public Health Preparedness for Disasters and Emergencies, and Forum on Microbial Threats (wow, say that 3 times fast!), this workshop has a broad-ranging agenda focused practical approaches to developing rapid, point-of-care diagnostics. Go here for details and to register.
- 19-23 Oct 2022 (Washington, DC): IDWeek 2022, the joint annual meeting of the Infectious Diseases Society of America (IDSA), Society for Healthcare Epidemiology of America (SHEA), the HIV Medicine Association (HIVMA), the Pediatric Infectious Diseases Society (PIDS), and the Society of Infectious Diseases Pharmacists (SIDP). Go here for details.
- 15-23 Oct 2022 (in person, residential, Les Pensières, Veyrier-du-Lac, France): The 6th edition of Patrice Courvalin’s fabulous ICARe residential training course covering all things AMR is on for 2022! This is a soup-to-nuts training in AMR: it is very intense, very detailed, and always gets rave reviews from attendees. Registration is open 21 Mar 2022 to 21 June 2022 and is limited, so book your slot as soon as you can. Go here for details.
- 19-23 Oct 2022 (Washington, DC): IDWeek 2022, the joint annual meeting of the Infectious Diseases Society of America (IDSA), Society for Healthcare Epidemiology of America (SHEA), the HIV Medicine Association (HIVMA), the Pediatric Infectious Diseases Society (PIDS), and the Society of Infectious Diseases Pharmacists (SIDP). Go here for details.
- 23 Oct 2022 (Cape Town, South Africa): Symposium entitled “Tackling AMR: How implementation research is vital in a One Health approach” sponsored by the AMR knowledge hub of TGHN (The Global Health Network). Go here for details.
- 25-28 Oct 2022 (Stellenbosch, South Africa): The University of Cape Town’s H3D Research Centre will celebrate its 10th anniversary with a symposium covering the Centre’s research on Malaria, TB, Neglected Tropical Diseases, and AMR. Go here to register.
- 17-20 Nov 2022 (Kuala Lumpur, Malaysia): The International Congress on Infectious Diseases will take place for the first time as a hybrid event. Go here for details.
- 27-30 Nov 2022 (Perth, Australia): 32nd International Congress of Antimicrobial Chemotherapy is the biennial congress of the International Society of Antimicrobial Chemotherapy (ISAC). Go here for details.
- 3-7 Dec 2022 (Banff, Canada): Novel Approaches Against Emerging Antimicrobial Resistance by Keystone Symposia. Go here for details.
- 8-12 May 2023 (Lisbon, Portugal): 41st Annual Meeting of the European Society for Paediatric Infectious Diseases. Go here for details.