Dear All (and with thanks to Damiano for taking the lead on this newsletter):
In advance of Italy assuming the G7 Presidency for 2024, The European House – Ambrosetti (an Italian think tank and management consulting firm) published a new report on 15 November 2023 entitled “Value and sustainability of antibiotics as essential tools for the healthcare system and people’s health.” The main objective of the report was to provide concrete recommendations to the Italian government about practical steps to address “the enormous clinical unmet need and the urgent need to make R&D of antibiotics more attractive and competitive, especially for Reserve-type antibiotics.”
The report concludes that “there is widespread consensus among experts in the medical and scientific community that no single incentive will be sufficient to stimulate antibiotic R&D and that, therefore, an approach that applies a combination of PUSH and PULL incentives throughout the antibiotic R&D pathway is necessary.” As a consequence, “it is crucial and urgent to adopt measures in Italy that promote innovation and support research to effectively address the antibiotic resistance challenges and sustain the development of new antibiotics.” We certainly agree with these conclusions!
Here are the links you’ll need. The full report is in Italian; there are good summaries in English:
- The report’s webpage
- The full report (in Italian)
- The press release (in English)
- A one-page infographic (in English)
- A one-page policy brief (in English)
- Deeper background: WHO’s Aware / Watch / Reserve antibiotic classification guidance
The 1-page infographic is very well done … the “discovery void” image is particularly excellent:
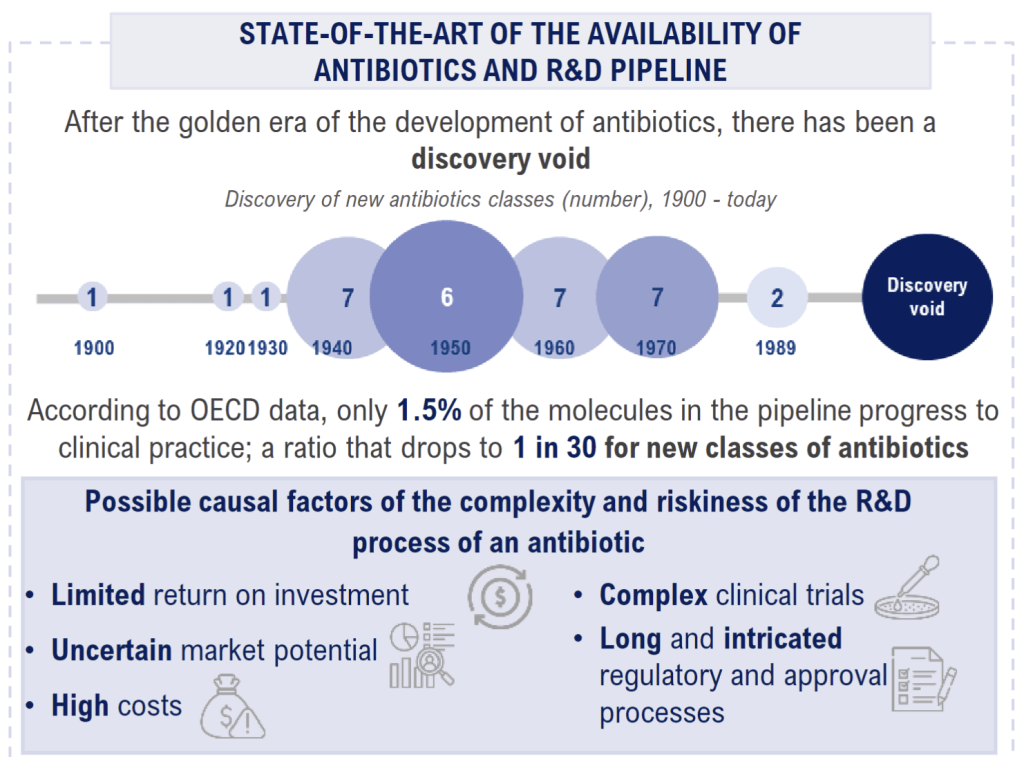
The report offers five concrete recommendations to the Italian government.
The first recommendation is about the leadership that the Italian government should use during its Presidency of the G7 in 2024 to ensure progress on complementary PUSH and PULL incentives at the global level:
- “Emphasize the issue of AMR within the agenda of the G7 during the Italian presidency in 2024, by promoting a pilot project on Pull incentives shared among G7 countries, complementary and not an alternative to existing Push incentives, strengthening the increasing collaboration between the respective regulatory agencies.”
- Note that this is in line with what the G7 Health Ministers said in Nagasaki in May 2023, where they committed to “exploring and implementing push and pull incentives that promote investment in R&D of antimicrobials, including contributing to existing global pooled efforts, such as CARB-X, GARDP and SECURE, at the earliest opportunity and within reasonable and feasible timelines.”
The second, third, and fourth recommendations suggest actions the Italian government could take to increase the rewards related to bringing innovative antibiotics to the Italian market via existing tools under the current administrative framework:
- “Establish dedicated and structural funding for Reserve antibiotics, using unallocated funds from the Innovative Medicines Fund, which has been increased in the 2022 Budget Law.”
- “Ensure that Reserve antibiotics receive a net ex-factory reimbursement price, currently practiced within the Italian NHS during the antibiotics’ stay in the Innovative Medicines Fund, for the entire exclusivity period. This encourages, following the English model, the appropriate use of antimicrobials (meaning the price should not be so high as to discourage appropriate use but not so low as to encourage misuse).”
- “Extend the period of innovativeness for Reserve antibiotics for a duration of at least 5 years, surpassing the current 36 months.”
The fifth recommendation proposes the development of a fully-fledged pull incentive, beyond the small tweaks to what already exists today covered by the other recommendations:
- “Create a new, ad hoc pathway for Reserve antibiotics by identifying, experimenting with, and introducing pricing and reimbursement models based on those adopted in other European and G7 countries. This recognition acknowledges the status of priority and innovation granted by the WHO definition.”
- This is a good moment to remind our readers that the most advanced estimate of the sufficient size for a fully delinked global subscription program (in addition to complementary global push incentives) is $310 million per drug annually over 10 years. A “fair share” for an Italian pull incentive would be a national pricing and reimbursement model rewarding an innovative, high-impact antibiotic with about €15 million annually over 10 years.
In conclusion, “it is essential to provide pharmaceutical companies with a new regulatory and policy framework favouring innovation, adapting existing policy tools (such as Innovation status) to the specificities of the sector and implementing a Push (encouraging the research and development of new antibiotics) and Pull (ensuring availability and access) incentives system.”
Wow! Yes, yes and yes! In the press release, Massimo Andreoni, Full Professor of Infectious Diseases at the faculty of Medicine and Surgery, University of Rome Tor Vergata, and Scientific Director, Italian Society of Infectious and Tropical Diseases (SIMIT), summarizes why the report is a long-awaited call to action for the Italian R&D and access landscape:
- “Despite the recent inclusion of antimicrobial resistance in the political agenda, with the publication of the second National Plan to Contrast Antimicrobial Resistance (PNCAR) 2022-2025 in February 2023, financed with €40 million for the years 2023, 2024, and 2025, and the presentation of a legislative proposal for the prevention and control of healthcare-associated infections often caused by multi-resistant pathogens, in Italy, there have not been impactful political-institutional actions to make antibiotics targeted at treating infections with limited treatment options, and classified as “Reserve” by the WHO, available and economically sustainable.”
Impressive and timely! After several positive steps towards the implementation of sustainable Push and Pull incentives from other G7 governments in the past few years (e.g., US here and here, UK here and here, Germany here and here, Japan here and here, Canada here and here), it is promising to see some movement in Italy as well, in particular given the fact that Italy will hold the G7 Presidency in 2024.
MANY thanks to the team at the European House – Ambrosetti for showing practical steps that Italy can take to sustain innovation and access to innovative antibiotics (and with thanks as well to Shionogi for the unconditional grant that supported creation of the report).
We look forward to Italy stepping up its efforts to address AMR as we progress towards the high-level meeting on AMR during UNGA 2024!
All best wishes,
Damiano & John
Damiano de Felice, PhD, Director of Development and External Engagement, CARB-X (these views are personal and do not necessarily reflect the views of CARB-X or any of this funders) @damidefelice
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- FDA have released a BAA covering a wide variety of regulatory topics. See this newsletter for general details; also note in particular an RFP for work on urine-specific breakpoints for uUTI. Early concept papers are due 6 Nov 2023; full proposals are due 19 Feb 2024.
- PACE (Pathways to Antimicrobial Clinical Efficacy), a £30 million initiative supporting early-stage innovation, has been created by jointly by Innovate UK, LifeArc, and Medicines Discovery Catapult (MDC). An initial round of up to £10 million in grant funding is available to support up to 12 projects focused on developing new treatments for the most threatening microbes and resistance mechanisms. Applications are welcomed from any part of the world for projects expected to last up to two years and with total funding of up to £1 million per project. Expressions of interest are invited by 24 November, and an informational webinar was held on 31 October 2023.
- ARPA-H have an Open BAA that is accepting applications through 14 March 2024. It is quite wide-ranging in its scope and definitely includes AMR-related projects. See this newsletter for discussion of the BAA and an AMR project that it now supports.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. Applications are accepted on a rolling basis; go here for all the details.
- The ENABLE-2 consortium has announced a call to support hit-to-lead compound development by researchers at publicly-funded European universities. The call is focused on molecules with the potential to be direct-acting therapies for one or more of the following priority pathogens: ESBL-producing/carbapenem-resistant Enterobacteriaceae (E. coli, K. pneumoniae), P. aeruginosa, A. baumannii, methicillin-resistant S. aureus, or vancomycin-resistant E. faecium. The Call is open continuously, applications are reviewed at intervals, and funding is non-dilutive. Expressions of interest received before 30 Sep 2023 would be considered in November 2023. Applications received after this date will be evaluated in the spring of 2024 (date to be decided). Go to https://www.ilk.uu.se/enable2/apply/ for further details.
- The AMR Action Fund is now open to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
John’s Top Recurring Meetings
Virtual meetings are easy to attend, but regular attendance at annual in-person events is the key to building your network and gaining deeper insight. My personal favorites for such in-person meetings are below. Of particular value for developers are the AMR Conference and the ASM-ESCMID conference. Hope to see you there!
- 6-7 Mar 2024 (Basel, Switzerland): The 8th AMR Conference 2024. Save the date! More details to come!
- 27-30 April 2024 (Barcelona, Spain): 34th ECCMID, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. Go here for details.
- 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. Go here for the meeting’s general website. You can’t register (yet) for the 2024 event, but save the date!
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. Save the date! More details to come!
Upcoming meetings of interest to the AMR community:
- 12 Dec 2023 (virtual, 1-2.15p ET): Duke-Margolis presents the third and final installment of their webinar series for policymakers combating AMR with a webinar entitled “Improving Regulatory Practices to Sustain Antibiotic Innovation.” The webinar will feature perspectives from experts affiliated with the FDA, Industry, GARDP, CARB-X, and the Center for Global Development. HIGHLY RECOMMENDED! Go here to register; prior webinars occurred on 9 May 2023 on the Need for New Antibiotics, and on 29 Aug 2023 on Preparedness for AMR Threats (also see their related October 2023 report on Preparedness and Post-Market Incentives for Novel Antibiotics).
- [NEW] 22 Jan 2024 (virtual, 5p-6.30p CET): ‘What do the various non-commercial actors in the antibiotics R&D ecosystem do?’ with Erin Duffy (CARB-X), Peter Beyer (GARDP), and Laura Marin (JPIAMR). Click here to register.
- 24 Jan 2024 (online, 9a-1p London): Webinar entitled “Priorities and next steps for tackling antimicrobial resistance in the UK”, sponsored by the Westminster Health Forum. Featured speakers include Dame Sally Davies and the focus is on the UK’s 20-year vision and 5-year action plan for AMR. Go here for details and to register.
- [UPDATED — registration link is active] 6-7 Feb 2024 (online): Antimicrobial Chemotherapy Conference. This is an annual, free of charge conference that is co-organized by GARDP and the British Society for Antimicrobial Chemotherapy (BSAC). Go here to register.
- [NEW] 8 Feb 2024 (in person, Liverpool, UK, 8.30a – 4p): 2024 BioInfect Conference. A full-day AMR conference that includes a keynote from Lord Jim O’Neill (Chairman of the UK AMR Review). Go here for details and to register.
- 6-7 Mar 2024 (Basel, 6-7 Mar 2024): See Recurring Meetings list, above.
- 17-22 Mar 2024 (Ventura Beach, CA, in person): Gordon Research Conference (GRC) entitled “New Antibacterial Discovery and Development” with a 16-17 Mar 2024 pre-conference Gordon Research Seminar (GRS) for young doctoral and post-doctoral researchers. An intensive residential meeting, GRCs are highly recommended for networking and deep research insights. Apply here for the GRC and here for the GRS.
- 27-30 April 2024 (Barcelona, Spain): 34th ECCMID, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. See Recurring Meetings list, above.
- 26-31 May 2024 (Montreal, Canada): EDAR7, the McGill AMR Centre’s 7th edition of their Environmental Dimension of Antimicrobial Resistance conference. Go here for details; final abstract deadline is 21 Dec 2023.
- 13-17 June 2024 (Atlanta, Georgia): ASM Microbe, the annual meeting of the American Society for Microbiology. You can’t register yet, but you can go here for general details.
- 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. See Recurring Meetings list, above.
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. See Recurring Meetings list, above.