Dear All (and with thanks to Kevin for co-authoring),
For today, a wonkish potpourri of data to support action. As you’ll see, this string of pearls is deeply connected:
- A UN Environment Programme (UNEP) report,
- An analysis of the global response to AMR during 2020-21 in 114 countries,
- A summary of the path to (and next steps for) the UK’s ground-breaking pilot subscription model for antibacterial Pull, and
- First sighting of a Pull incentive in Japan!
1. First up, a just-released report from UNEP entitled “Bracing for Superbugs: Strengthening environmental action in the One Health response to antimicrobial resistance.” Released during the 6th Meeting of the Global Leaders Group on AMR (GLG6) in Barbados (6-7 Feb 2023), the report brings an important environmental perspective to the AMR problem by providing evidence that environment matters in the development and spread of AMR. The heart of the report is a review in Section 3 of the mechanisms of environmental spread of AMR. There are some great figures here … see examples below the signature block. Landfill run-off, migratory birds, SDGs, and more!
The report calls for a multisectoral One Health response of priority actions to address key pollution sources from poor sanitation; sewage; community and municipal wastes; healthcare delivery; pharmaceutical manufacturing; intensive crop farming; and terrestrial and aquatic animal production sectors. Overall, a well-written and timely report with something to learn on almost every page … we would encourage even jaded AMR report readers to set aside an hour for this one!
—
2. Reports of the type released by UNEP are a big deal because global UN reports create momentum at the political level. On this theme, our 2nd stop in today’s tour takes us to an analysis of the impact of calls to date for action AMR.
In a paper by Patel et al. entitled “Measuring the global response to antimicrobial resistance, 2020–21: a systematic governance analysis of 114 countries,” researchers at Edinburgh’s Global Health Governance Programme reviewed the contents of NAPs (National Action Plans) on AMR from around the world.
You are probably aware that WHO have been pushing countries to implement NAPs since 2015 — go here to see a library of current NAPs and a handbook for creating a NAP. In addition, WHO, FAO, and OIE have been monitoring NAPs since 2016 via an annual Tripartite AMR country self-assessment survey (TrACSS). Data provided in these surveys is shared in the open tripartite database and gives a good sense of # of NAPs.
Building on the theme of tracking implementation, Patel et al. have combined data from TrACSS, the published NAPs, and surveillance data from GLASS (the Global Antimicrobial Resistance and Use Surveillance System) to compute a by-country overall AMR governance score for the period 2020-21 based on assessment across the domains of (i) policy design, (ii) implementation tools, and (iii) monitoring and evaluation. Here’s the resulting display of governance scores:
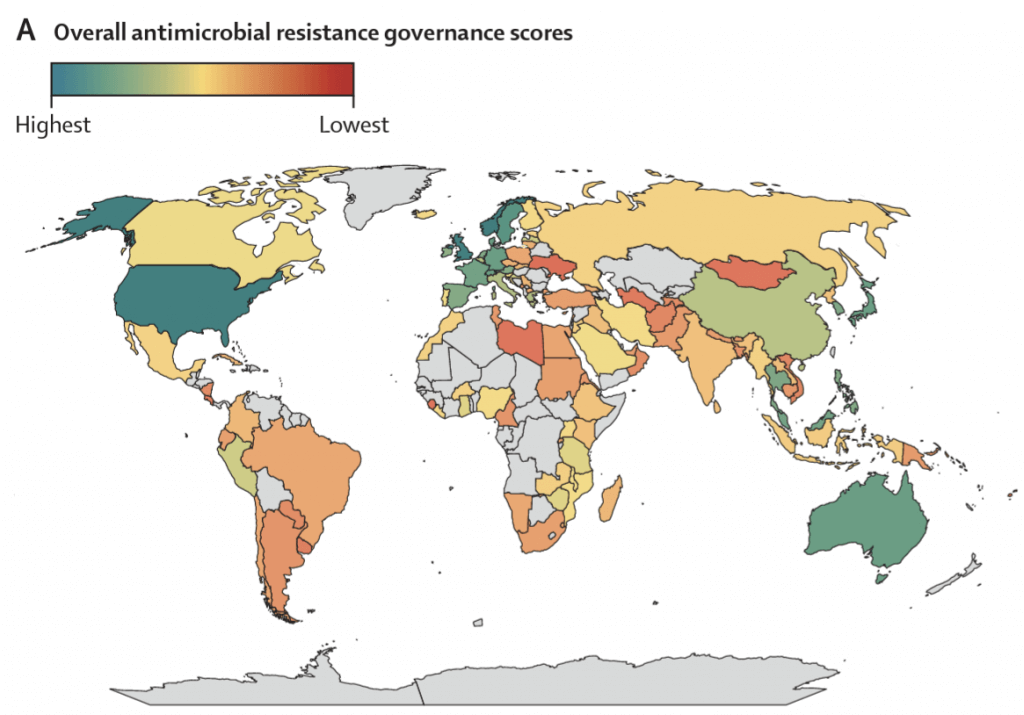
Excerpt from Figure 3 from Patel et al. Map of global governance scores 2020-21. See also a more detailed set of graphics below the signature block.
From the figure, you can readily see both the range of governance scores and the regions for which data are/are not available. By providing this level of comparability, we can move beyond simply counting the number of published NAPs to assessing areas for improvement and global cooperation.
—
3. Our 3rd stop takes us to a very specific question of implementation: Pull incentives.
The most advanced such work is the ground-breaking UK subscription model that has now selected two antibiotics to purchase on a volume-delinked basis. To summarize their experience with the pilot, the UK implementation team have published “Can the UK ‘Netflix’ Payment Model Boost the Antibacterial Pipeline?” (Leonard, C., Crabb, N., Glover, D. et al., Appl Health Econ Health Policy https://doi.org/10.1007/s40258-022-00786-1, 2023).
The paper by Leonard et al. is a great tour of the timeline of the project, and in particular you get an insider’s view of the key challenges encountered during the project. The big ones reviewed by the authors were:
- Building political, health system, and industry support and in particular overcoming initial misconceptions that the project set out to reward big pharma.
- Adjusting HTA methodology to more adequately assess the additional attributes of value brought by new antibacterials.
- Developing selection criteria and a transparent scoring system to choose two antibacterials for this new approach.
- Finally, implementing a novel HTA process to value the selected candidate products.
Kevin Outterson and I had the opportunity to write an editorial to accompany the paper. In it, we (i) summarize the calls to action from all sectors (government, academia, civil society, think tanks, and more), (ii) review briefly the STEDI principles that underpin the HTA process used by the UK team (for a refresher on STEDI, see this newsletter or this YouTube explainer), and (iii) consider and respond to critiques of the approach. In particular, we emphasize the need to communicate clearly that substantial Pull incentives won’t apply to every antibacterial, but will be used both pragmatically and selectively to reward timely innovation. We also make several suggestions for the next phases of the UK’s work.
Fingers crossed that we’ll see further action to implement Pull incentives. The PASTEUR Act continues to be considered in the United States (we came close to getting it passed in late 2022 and will try again in 2023), and there are ongoing discussions in the EU and other parts of the world.
4. As breaking news from the 6th Global Leaders Group meeting (GLG6, Barbados, 6-7 Feb 2023), we have the first sighting of a Pull incentive in Japan! Details are pending, but study of the slide shared at GLG6 reveals an FY2023 budget proposal of 1.1b yen ($8.3m) and subsidy-based design. Although that initial budget looks small relative to the economy of Japan, the intent appears to be a delinked incentive that is large enough to provide an actual innovation incentive.
—
Exciting stuff! Although it can feel like things move at a glacial pace, the continued efforts of stakeholders around the world are gradually moving the needle. Many thanks to the Global Leaders Group for pressing for political action … we look forward to a UN High Level Meeting on AMR that we’re told will be held in NYC in Sept 2024.
“It always seems impossible until it is done.” — variously attributed.
All best wishes, John & Kevin
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Kevin Outterson, JD, Professor of Law, Boston University & Executive Director, CARB-X (these views are personal and do not necessarily reflect the views of CARB-X or any of its funders) @koutterson
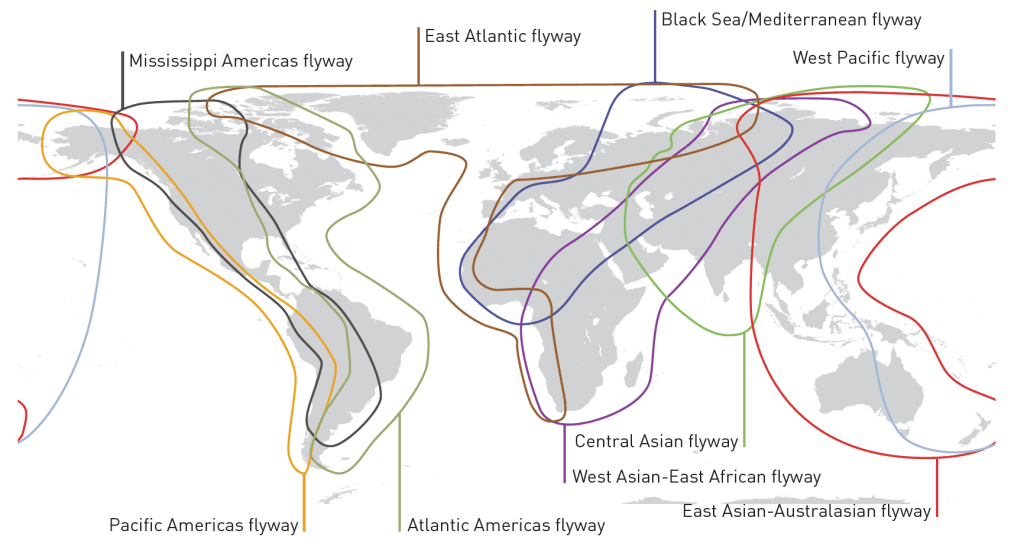
Figure 11 from the UNEP report: Bird migration pathways. Yes, birds can spread AMR! As discussed on page 20 of the report, the first reported occurrence of carbapenemase-producing bacteria (NDM-1 enzyme in Salmonella enterica) in a wild animal were isolated from black kites (Milvus migrans) in Germany in 2013 (Fischer et al., 2013).
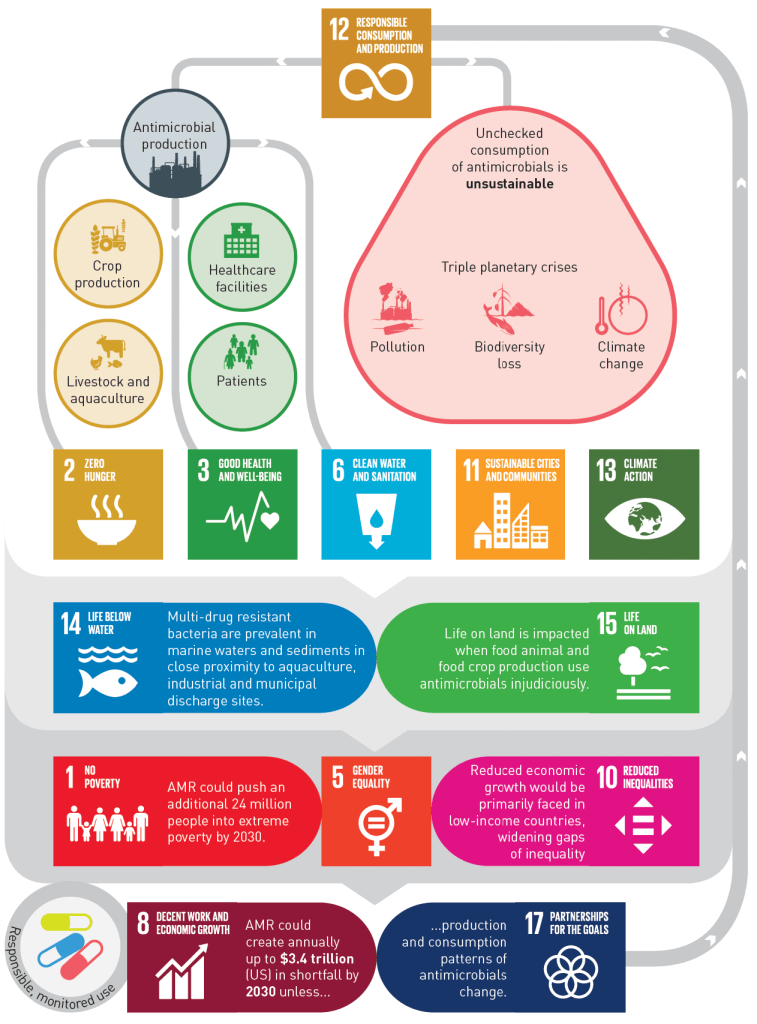
Figure 13 from the UNEP report: How sustainable production and consumption of antimicrobials impacts 13 of the UN’s 17 SDGs (Sustainable Development Goals). The only ones missing are #4 (Quality Education), #7 (Affordable and Clean Energy), #9 (Industry, Innovation, and Infrastructure), and #16 (Peace, Justice, and Strong Institutions).

Figure 3 from Patel et al. Map of global governance scores 2020-21 (upper left) and then by domains of Policy Design, Implementation Tools, and Monitoring and Evaluation. Overall, you can see that Domains relating to policy design and implementation tools generally score similarly and higher than Monitoring and Evaluation.
Current funding opportunities (most current list is here)
- JPIAMR call for projects on AMR Diagnostics and Surveillance. Under the collaborative umbrella of ERA-NET, this JPIAMR-ACTION project seeks development of innovative strategies, tools, technologies, and methods for diagnostics and surveillance of antimicrobial resistance. The project involves 23 funders from 19 countries and the total estimated call budget is about 19,1 million Euro. Go here for the full webpage on the call. Pre-proposals are due by 7 Mar 2023.
- NIAID BAA with a biodefense focus: Vaccines, Therapeutics, and Diagnostics are all in scope. See this newsletter for details; various due dates through 11 April 2023.
- Current funding rounds from CARB-X are as described in this newsletter!
- The AMR Action Fund is now open to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- BARDA’s long-running BAA-18-100-SOL-00003 offers support for both antibacterial and antifungal agents. This BAA has offered 4 deadlines/year since 2018 … check the most current amendment for details.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- 14 Feb 2023 (virtual, noon-1.30p CET): Webinar entitled “WHO People-centred framework on addressing AMR” from WHO’s series entitled “WHO Global Webinar Series to Support Implementation of National Action Plans on Antimicrobial Resistance (AMR).” Go here to register.
- 2 Mar 2023 (virtual, 1.00-2.35p ET): Webinar from Duke-Margolis entitled “Data Capture to Measure, Track, and Improve Antibiotic Use.” The panel discussion at this webinar will seek to inform possible policy approaches that could be used as part of incentives for novel antibiotics. Timely! The meeting’s website is here; go here to register.
- 16 Mar 2023 (virtual, noon-1.30p CET): Webinar entitled “WHO AMR Costing & budgeting tool: A review and country experiences” from WHO’s series entitled “WHO Global Webinar Series to Support Implementation of National Action Plans on Antimicrobial Resistance (AMR).” Go here to register.
- 16-17 Mar 2023 (timings suggest hybrid EU-US): 7th AMR Conference, hosted by the BEAM Alliance with many co-sponsors. This has historically been a very good networking event. Go here for details.
- 23-24 March 2023 (virtual, 10a-4p ET): 23rd Meeting of the Presidential Advisory Council on Combating Antibiotic Resistance (PACCARB). The PACCARB will vote on the report from the Pandemic Preparedness Working Group (PPWG) on how to strengthen defenses against AMR pathogens in the face of a potential future, large-scale disease event. Go here for details and to register.
- 14 Apr 2023 (Copenhagen, Denmark; 3-6.30p CEST): ECCMID and the Global Leaders Group on AMR will jointly sponsor a symposium entitled “Forging partnerships between science and policy in Antimicrobial Resistance (AMR).” Go here to register.
- 15-18 Apr 2023 (Copenhagen, Denmark): 33rd ECCMID. Go here for details and to register.
- 26 Apr 2023 (virtual, noon-1.30p CET): Webinar entitled “WHO Human health AMR research agenda” from WHO’s series entitled “WHO Global Webinar Series to Support Implementation of National Action Plans on Antimicrobial Resistance (AMR).” Go here to register.
- 8-12 May 2023 (Lisbon, Portugal): 41st Annual Meeting of the European Society for Paediatric Infectious Diseases. Go here for details.
- 7-15 Oct 2023 (residential, Annecy, France): ICARe, the Interdisciplinary Course on Antibiotics and Resistance. Now in its 7th year, this course is a deep-dive into the world of antibiotic development. Intense, rigorous, and HIGHLY recommended. Seats are always limited … apply sooner rather than later! Go here for details.
- 20-23 Oct 2023 (Athens, Greece): 11th TIMM (Trends in Medical Mycology). Go here for details.