Dear All,
In a report entitled Understanding the Antibiotic Manufacturing Ecosystem: A view of global supply chains, pressure points, and implications for antimicrobial resistance response, the Boston Consulting Group and Wellcome Trust have examined at length the questions of (i) how the #FireExtinguishersOfMedicine are made and (ii) how they can be made with the least impact on the environment.
To see all this in context, there are are two parallel threads to consider. First, COVID has taught us about the fragile nature of supply chains in general. Antibiotics definitely have fragile supply chains and you can get a flavor of this by scanning this 2018 article in Forbes, this 2021 paper in BMJ Global Health, and FDA’s Drug Shortage webpage. Second, manufacturing discharges can themselves drive antibiotic resistance — ATMF’s 2021 review is a good summary of the problem. The need for responsible approaches to manufacturing was also clearly called out in proposals to establish environmental discharge from the AMR Industry Alliance. Notably, the AMR Industry Alliance (AMRIA) will publish an industry standard next month which, together with an independent certification scheme (expected in early 2023), could provide a mechanism to drive adoption of risk based, cost effective controls to minimize antibiotic emissions across the global supply chain and provide means by which manufacturers can give assurance of responsibly made antibiotics.
Addendum: the AMRIA published its Antibiotic Manufacturing Standard on 14 June 2022. See https://www.amrindustryalliance.org/shared-goals/common-antibiotic-manufacturing-framework/ for details, including research-based Predicted No-Effect Concentrations (PNECs). In collaboration with BSI (British Standards Institution), there is also an industry certification scheme.
So, this new report analyzes the impact of possible environmental regulatory changes on antibiotic supply. The report starts from a very practical concern — it regulatory changes increase manufacturing costs, will manufacturing decline?
There are some excellent figures throughout the report but I wanted to share these two in particular. Production of antibiotics is not simple and, unfortunately, antibiotic discharge does happen. Note in particular (2nd figure) that most of the manufacturing happens in two of the most densely populated countries in the world:
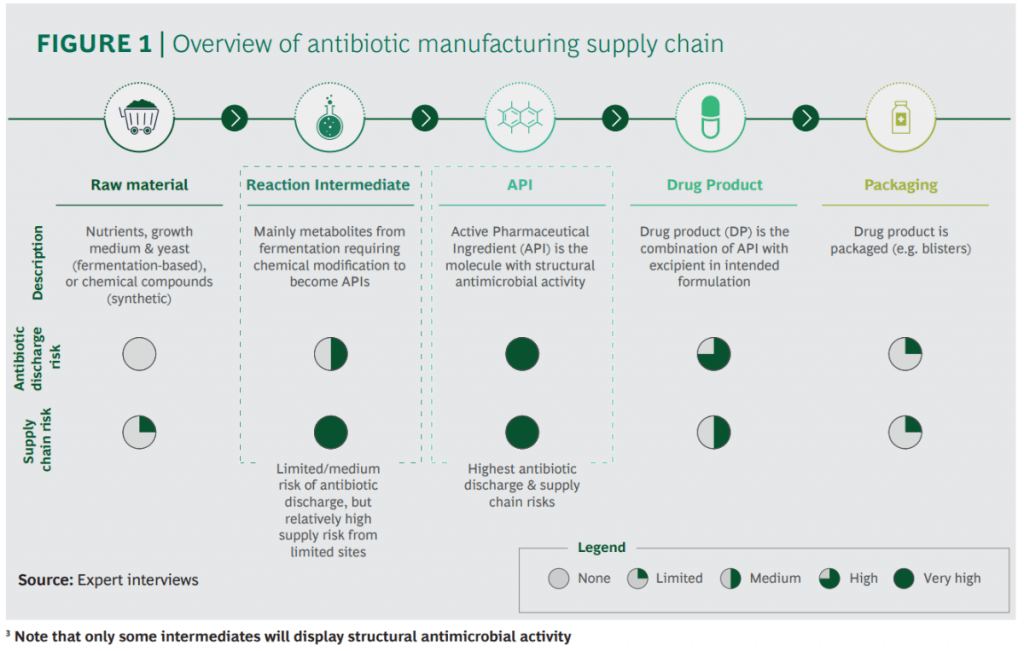
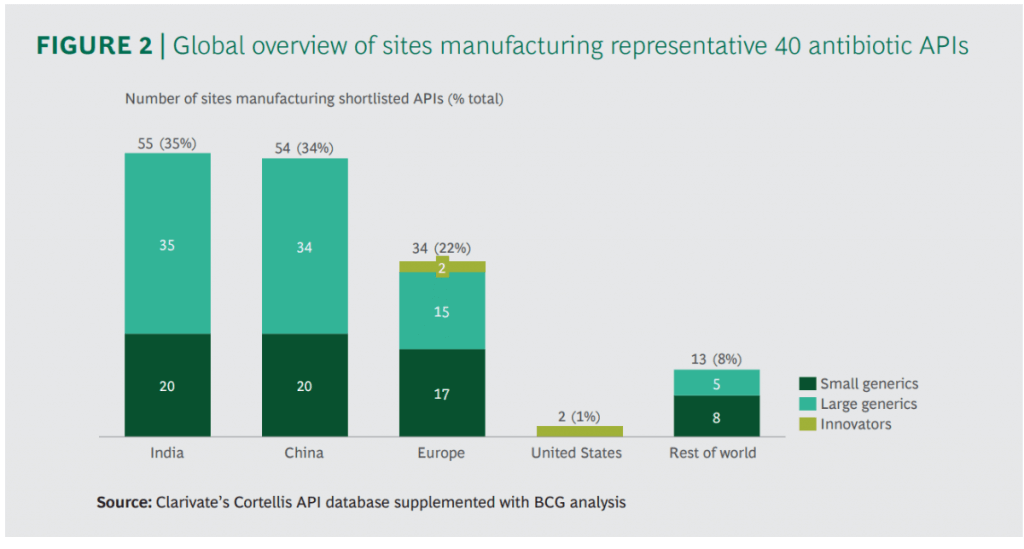
In terms of the impact of environmental regulation to curb this issue, it really boils down to someone paying more and at what scale. If it is the developers, we all know how tough it is to just keep the lights on once you’ve made a new antibiotic. If it is the consumers, no one ever wants to pay more if they can help it. And, none of us will be happy if we break the supply chains for critical antibiotics! But how likely is this if new environmental regulation was introduced?
I encourage you to read the report yourself, but findings suggest environmental regulation can be compatible with strong antibiotic supply chains. However, policies should be developed in a supply chain-sensitive way and complimented with supportive measures for at-risk supply chains. The key being (no surprise!) to take a number of pragmatic steps in parallel. These include:
- Evidence-based discharge limits
- Standardized methods for monitoring discharge
- Cost-efficient processes for meeting and monitoring compliance with the targets
- Ambitious but feasible timelines for implementation
- Consideration of the fragility of individual supply chains with an eye to ensuring that supply chains for critical antibiotics are not broken
- International coordination on all the above, including actions by net-importers
The report suggests that the WHO and G7/G20 need to take the lead: global government action will be needed to change the way we develop and use antibiotics. Now is the time to include changing the way we manufacture them as part of this global effort!
Well done, BCG and Wellcome Trust! –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Current funding opportunities (most current list is here):
- JPIAMR’s 14th call is now open. Entitled “Disrupting drug resistance using innovative design”, the call seeks consortia that would seek to “improve the treatment of bacterial and fungal infections (including co-infection) and/or the prevention of the emergence/spread of resistance in humans, animals or plants through the improvement of the efficacy, specificity, delivery, combinations and/or repurposing of drugs and plant protection agents.” Bacteria, fungi, human health, animal health, and plant health are all in scope! Pre-proposals are due 8 Mar 2022; full proposals would be due 5 July 2022. Go here for details.
- The AMR Action Fund is now open to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is a newly launched early-stage funding vehicle. Details are still coming into focus, but per comments on 25 Aug 2021 at the BIOCOM conference, their goal is to support ~4 companies per year with about $250k/company. Contact details are on their website (https://www.incate.net/).
- CARB-X recently announced that their existing resources will be reserved to fund their existing portfolio (more than 80 total awards, and counting, as they include contracting from prior rounds). New rounds from CARB-X will occur only after new funding is obtained in 2021.
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- 8 Dec 2021: “The New Winds Pushing and Pulling Antibacterial Development.” This FABULOUS program featured talks from the UK team behind the NHS “Netflix” pilot, Kevin Outterson’s recently released report documenting the need for global Pull incentives to have a value of $2.2 – 4.8b, and speakers covering PASTEUR and work in the EU on pull incentives. The video is here — please make time to listen to this program!
- The stunning 4 Feb 2022 webinar for the GRAM report (Global Research on Antimicrobial Resistance “1.27 million deaths per year are directly attributable to AMR”) is now available for replay. #AMRSOS!
- [NEW] 13 May 2022 (Strasbourg, France): AC21 Workshop: “Pre-clinical development of antimicrobial peptides”, with contributions from the AC21 member universities in Stellenbosch, South Africa, Minneapolis, USA, Freiburg, Germany and Strasbourg, France as well as from other institutions. Participation is free and open to non-member institutions but registration is required. Supported by Academic Consortium 21. Go here for details.
- 16-18 June 2022 (Perth, Australia): Australasian Society for Infectious Diseases Annual Scientific Meeting is a hybrid event for adult and pediatric infectious disease and clinical microbiology specialists. Go here for details.
- 11-14 July 2022 (Sydney): Australian Society for Microbiology Annual National Meeting is a hybrid event that will feature a range of lectures and symposium sessions, as well as extensive opportunities for networking. Go here for details.
- 24-27 July 2022 (Il Ciocco, Tuscany): Gordon Research Conference entitled “New Antibacterial Discovery and Development”. Go here for details, go here for the linked 5-6 Mar Gordon Research Seminar that precedes it.
- 28-31 July 2022 (Singapore): 10th International Congress of Asia Pacific Society of Infection Control is a hybrid event for professionals in the Asia Pacific region. Go here for details.
- 20-24 Sep 2022 (New Delhi): 21st Congress of the International Society for Human and Animal Mycology (ISHAM). Go here for details.
- [NEW] 4-7 Oct 2022 (Dublin, Ireland): The 2022 ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. This is an excellent meeting, especially for developers … and if you’ve missed it, the recordings from the 2021 meeting are online. Go here for details on the 2022 meeting.
- 19-23 Oct 2022 (Washington, DC): IDWeek 2022, the joint annual meeting of the Infectious Diseases Society of America (IDSA), Society for Healthcare Epidemiology of America (SHEA), the HIV Medicine Association (HIVMA), the Pediatric Infectious Diseases Society (PIDS), and the Society of Infectious Diseases Pharmacists (SIDP). Go here for details.
- 15-23 Oct 2022 (in person, residential, Les Pensières, Veyrier-du-Lac, France): The 6th edition of Patrice Courvalin’s fabulous ICARe residential training course covering all things AMR is on for 2022! This is a soup-to-nuts training in AMR: it is very intense, very detailed, and always gets rave reviews from attendees. Registration is open 21 Mar 2022 to 21 June 2022 and is limited, so book your slot as soon as you can. Go here for more: https://www.icarecourse.org/
- 19-23 Oct 2022 (Washington, DC): IDWeek 2022, the joint annual meeting of the Infectious Diseases Society of America (IDSA), Society for Healthcare Epidemiology of America (SHEA), the HIV Medicine Association (HIVMA), the Pediatric Infectious Diseases Society (PIDS), and the Society of Infectious Diseases Pharmacists (SIDP). Go here for details.
- 25-28 Oct 2022 (Stellenbosch, South Africa): The University of Cape Town’s H3D Research Centre will celebrate its 10th anniversary with a symposium covering the Centre’s research on Malaria, TB, Neglected Tropical Diseases, and AMR. Go here to register.
- 27-30 Nov 2022 (Perth, Australia): 32nd International Congress of Antimicrobial Chemotherapy is the biennial congress of the International Society of Antimicrobial Chemotherapy (ISAC). Go here for details.