Dear All: Three recent papers bring together an instructive perspective on Pull incentives for the #FireExtinguishersOfMedicine. The first two papers survey the available tools and conclude that subscription models (e.g. “Netflix”) and transferable exclusivity vouchers (TEVs) are the key paths forward. The 3rd paper is new (and big) news: it shows that the existence of such Pull rewards would unlock significant private capital investment and thereby multiply the effect of the Pull reward! Here we go:
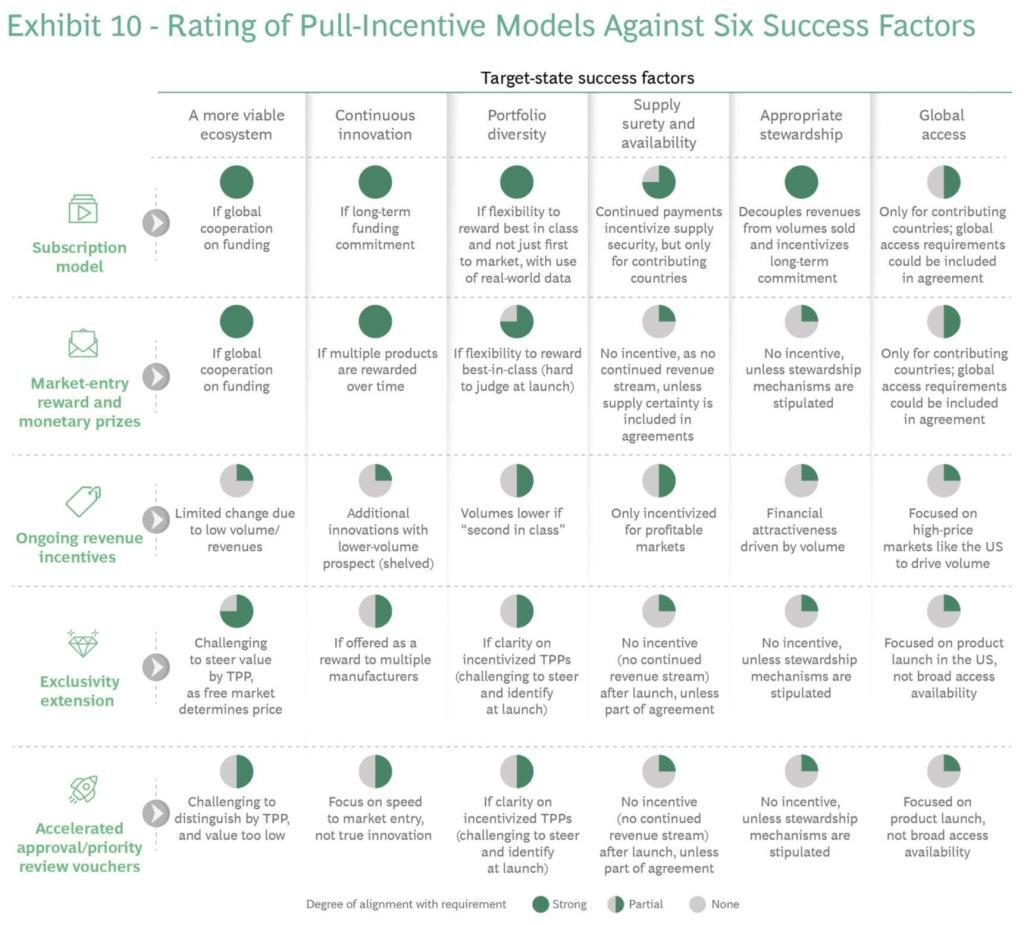
- Boston Consulting Group: The Case for a Subscription Model for Tackling Antimicrobial Resistance. This paper surveys a variety of Pull models (see figure below my signature) and concludes that all models have their strengths, Subscriptions models (e.g., the “Netflix” concept that is basis of the PASTEUR Act in the US) have the broadest applicability.
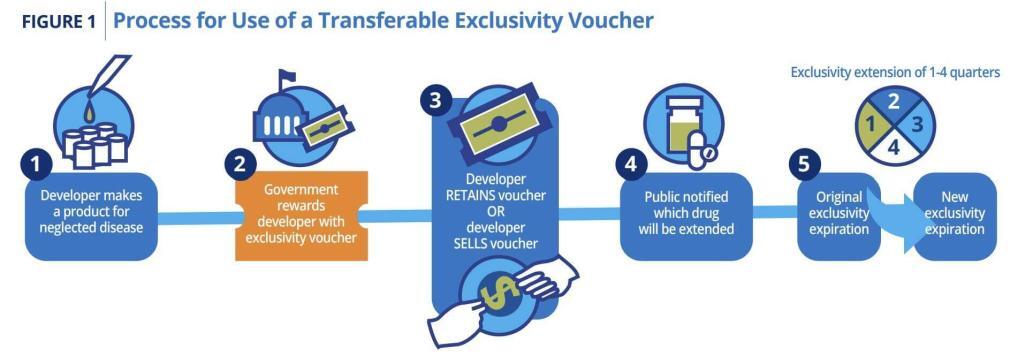
- Duke Margolis Center for Healthcare Policy: Design of a Transferable Exclusivity Voucher Program. Transferable Exclusivity Vouchers (TEVs) have long also tantalized because of their simplicity — the antibiotic is rewarded via the patent on another agent (see figure below my signature). This alternative to Subscription models may be just the thing for some territories.
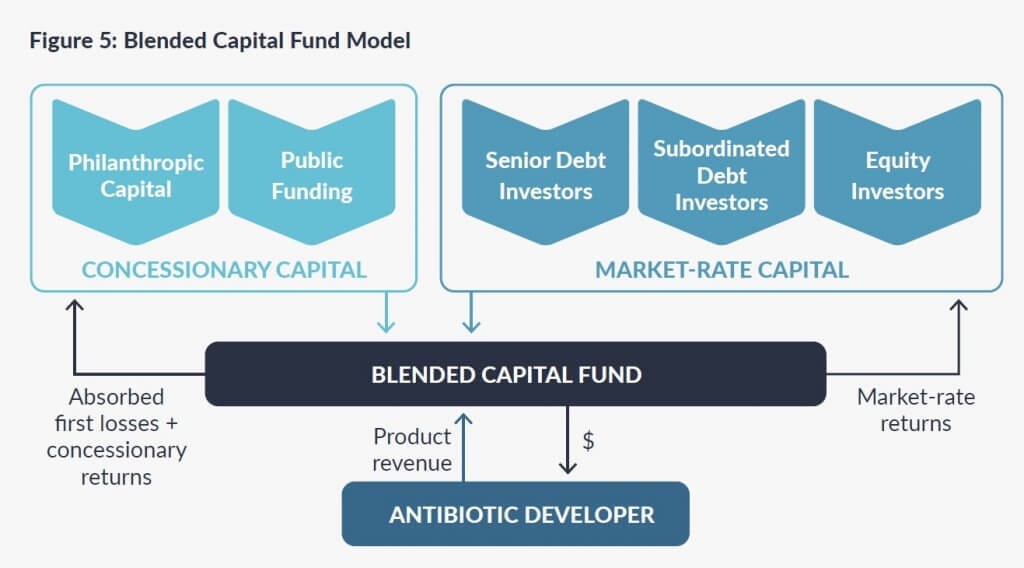
- Milken Institute: Models for Financing Antibiotic Development to Address Antimicrobial Resistance. The Milken Institute sought ways to involve private capital in AMR via a series of workshops during late 2021. Via this process, they have identified two ways that Pull rewards would cause private capital to invest, hence multiplying the impact of the Pull reward (see figures below my signature):
- A blended capital fund offering financing adjusted to the changing capital needs of an antibiotic as it moves through development.
- An “antibiotic bond,” in which committed subscription payments would provide the capital to pay back investors.
It’s exciting to have this expanded thinking. It confirms the findings of DRIVE-AB that (i) there are relatively few viable incentives tools (subscription models such as PASTEUR [Netflix] or TEVs consistently float to the top) and (ii) private capital will step in to multiply the effect of Pull incentives such as PASTEUR or a TEV. The idea of the potential for a multiplier effect from private capital is a novel observation and is something we need to emphasize in our messages. As an example, the Milken paper estimates a 2-3x multiplier effect for their blended capital fund concept .
Yow! All this really depends on Pull incentives such as PASTEUR … but it really could work!
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
—
Key figure from the BCG report:
Key figure from the Duke Margolis paper:
Key figures from the Milken Institute paper: Blended Capital and Pooled Loan models:

Current funding opportunities (most current list is here):
- BARDA is seeking proposals under Project BioShield to support late stage development, regulatory approval and potential procurement of antibiotics against biothreat pathogens, Yersinia pestis, Francisella tularensis, and Burkholderia pseudomallei. Small businesses can apply until April 6, 2022. Go here for the details.
- JPIAMR’s 14th call is now open. Entitled “Disrupting drug resistance using innovative design”, the call seeks consortia that would seek to “improve the treatment of bacterial and fungal infections (including co-infection) and/or the prevention of the emergence/spread of resistance in humans, animals or plants through the improvement of the efficacy, specificity, delivery, combinations and/or repurposing of drugs and plant protection agents.” Bacteria, fungi, human health, animal health, and plant health are all in scope! Pre-proposals are due 8 Mar 2022; full proposals would be due 5 July 2022. Go here for details.
- The AMR Action Fund is now open to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is a newly launched early-stage funding vehicle. Details are still coming into focus, but per comments on 25 Aug 2021 at the BIOCOM conference, their goal is to support ~4 companies per year with about $250k/company. Contact details are on their website (https://www.incate.net/).
- CARB-X recently announced that their existing resources will be reserved to fund their existing portfolio (more than 80 total awards, and counting, as they include contracting from prior rounds). New rounds from CARB-X will occur only after new funding is obtained in 2021.
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- 8 Dec 2021: “The New Winds Pushing and Pulling Antibacterial Development.” This FABULOUS program featured talks from the UK team behind the NHS “Netflix” pilot, Kevin Outterson’s recently released report documenting the need for global Pull incentives to have a value of $2.2 – 4.8b, and speakers covering PASTEUR and work in the EU on pull incentives. The video is here — please make time to listen to this program!
- The stunning 4 Feb 2022 webinar for the GRAM report (Global Research on Antimicrobial Resistance “1.27 million deaths per year are directly attributable to AMR”) is now available for replay. #AMRSOS!
- [NEW] 5 Apr 2022 (virtual): WHO-sponsored webinar, “Understanding options for World Bank financing to address AMR.” This webinar is part of the WHO Global Webinar Series to support implementation of national action plans on antimicrobial resistance (AMR). Go here to register.
- 7-8 Apr 2022 (Basel): The 6th edition of the annual AMR conference sponsored by the BEAM Alliance, CARB-X, the Novo REPAIR Impact Fund, the IMI Accelerator, and the European Biotechnology Network. Go here for the hold-the-date page and a way to be kept informed about the meeting.
- 23-26 Apr 2022 (Lisbon): 32nd European Congress of Clinical Microbiology & Infectious Diseases (ECCMID). Go here for details.
- 9-13 May 2022 (Athens and online): 40th Annual Meeting of the European Society for Paediatric Infectious Diseases, Go here for details.
- 20-24 Sep 2022 (New Delhi): 21st Congress of the International Society for Human and Animal Mycology (ISHAM). Go here for details.
- 15-23 Oct 2022 (in person, residential, Les Pensières, Veyrier-du-Lac, France): The 6th edition of Patrice Courvalin’s fabulous ICARe residential training course covering all things AMR is on for 2022! This is a soup-to-nuts training in AMR: it is very intense, very detailed, and always gets rave reviews from attendees. Registration is open 21 Mar 2022 to 21 June 2022 and is limited, so book your slot as soon as you can. Go here for more: https://www.icarecourse.org/
- 19-23 Oct 2022 (Washington, DC): IDWeek 2022, the joint annual meeting of the Infectious Diseases Society of America (IDSA), Society for Healthcare Epidemiology of America (SHEA), the HIV Medicine Association (HIVMA), the Pediatric Infectious Diseases Society (PIDS), and the Society of Infectious Diseases Pharmacists (SIDP). Go here for details.
- 25-28 Oct 2022 (Stellenbosch, South Africa): The University of Cape Town’s H3D Research Centre will celebrate its 10th anniversary with a symposium covering the Centre’s research on Malaria, TB, Neglected Tropical Diseases, and AMR. Go here to register.